研究方向
我們課題組長期從事天然免疫和線粒體質(zhì)量調(diào)控等方向的基礎(chǔ)研究,聚焦在DNA天然免疫信號(hào)通路、PINK1-Parkin信號(hào)通路、自身免疫性疾病、細(xì)菌與炎癥等領(lǐng)域。實(shí)驗(yàn)室綜合運(yùn)用細(xì)胞生物學(xué)、結(jié)構(gòu)生物學(xué)、生物化學(xué)和免疫學(xué)方法研究天然免疫、線粒體調(diào)控領(lǐng)域的核心機(jī)理和潛在應(yīng)用。現(xiàn)階段的研究工作主要集中于:1)線粒體的質(zhì)量調(diào)控與天然免疫的調(diào)控機(jī)制;2)細(xì)菌的環(huán)狀核苷酸代謝通路與免疫的調(diào)控機(jī)理;3)上述機(jī)制、過程在炎癥、自身免疫性疾病和神經(jīng)退行性疾病治療中的可能應(yīng)用。
科學(xué)貢獻(xiàn)
PINK1-Parkin信號(hào)通路與神經(jīng)退行性疾病帕金森癥密切相關(guān),我們系統(tǒng)研究了PINK1激活Parkin的核心作用機(jī)理,發(fā)現(xiàn)了Parkin被激活的正反饋機(jī)制。在DNA天然免疫領(lǐng)域,我們的工作主要集中在cGAS-STING信號(hào)通路的下游核心蛋白STING的激活機(jī)理。我們的研究系統(tǒng)闡述了STING作為cGAS-STING信號(hào)通路下游的核心蛋白,結(jié)合cGAMP后激活的全面的分子機(jī)制。我們的工作,為STING相關(guān)的自身免疫性疾病的治療和小分子激活劑和抑制劑的開發(fā),奠定了理論基礎(chǔ)。
研究成果
當(dāng)線粒體發(fā)生損傷時(shí),機(jī)體能夠通過PINK1-Parkin信號(hào)通路來誘導(dǎo)線粒體自噬(mitophagy),將損傷的線粒體及時(shí)地清理掉。PINK1和Parkin的失活突變是早期帕金森癥的重要致病原因。在PINK1-Parkin信號(hào)通路調(diào)控領(lǐng)域,我們在細(xì)胞、生化以及分子水平上證明了磷酸激酶PINK1作為Parkin的上游蛋白,激活Parkin的核心機(jī)理 (圖1 )。

圖1:PINK1激活Parkin并調(diào)控細(xì)胞命運(yùn)的機(jī)制
cGAS-STING通路是主要的DNA天然免疫信號(hào)通路。STING蛋白是該信號(hào)通路下游的核心接頭蛋白。我們的研究工作結(jié)合結(jié)構(gòu)和功能,系統(tǒng)闡述了STING結(jié)合第二信使cGAMP之后,形成聚集,激活TBK1-STING-IRF3磷酸化信號(hào)通路的全面分子機(jī)制(圖2)。
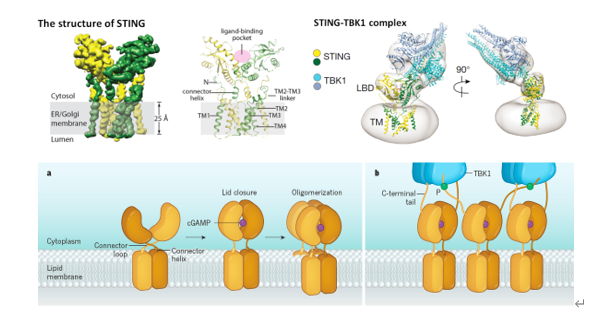
圖2:STING蛋白的結(jié)構(gòu)基礎(chǔ)和STING-TBK1通路的激活機(jī)制
代表性論文
1.
Zhang C?, Shang G?, Gui X, Bai X, Zhang X, Chen J. Z. (2019). Structural Basis of STING Binding with and Phosphorylation by TBK1.
Nature. 567: 394-398.
2.
Zhang C, Wang R, Liu Z, Bunker E, Lee S, Giuntini M, Chapnick D, and Liu X. (2019). The plant triterpenoid celastrol blocks PINK1-dependent mitophagy by disrupting PINK1’s association with the mitochondrial protein TOM20.
J Biol Chem. 294: 7472-7487.
3. Shang G ?,
Zhang C ?, Chen J. Z, Bai X, Zhang X. (2019). Cryo-EM Structures of STING Reveal Its Mechanism of Activation by Cyclic GMP-AMP.
Nature. 567: 389-393.
4.
Zhang C ?, Liu Z ?, Bunker E ?, Ramirez A, Lee S, Peng Y, Tan AC, Eckhardt SG, Chapnick DA, Liu X. (2017). Sorafenib Targets the Mitochondrial Electron Transport Chain Complexes and ATP Synthase to Activate the PINK1-Parkin Pathway and Modulate Cellular Drug Response.
J Biol Chem. 292: 15105-15120.
5.
Zhang, C., Lee, S., Peng, Y., Bunker, E., Shen, C., Giaime, E., Shen, J., Shen, J., Zhou, Z., and Liu, X. (2015). A chemical genetic approach to probe the function of PINK1 in regulating mitochondrial dynamics.
Cell research. 25(3):394-7.
6.
Zhang, C., S. Lee, Y. Peng, E. Bunker, E. Giaime, J. Shen, Z. Zhou, and X. Liu. (2014). PINK1 Triggers Autocatalytic Activation of Parkin to Specify Cell Fate Decisions.
Curr Biol. 24:1854-65.
7. Lee, S ?.,
Zhang, C ?., and Liu, X. (2014). Role of Glucose Metabolism and ATP in Maintaining PINK1 Levels During Parkin-mediated Mitochondrial Damage Responses.
J Biol Chem. 290(2):904-17.
8. Liu, S.,
C. Zhang, T. Su, T. Wei, D. Zhu, K. Wang, Y. Huang, Y. Dong, K. Yin, S. Xu, P. Xu, and L. Gu. (2014). Crystal structure of DszC from
Rhodococcus sp. XP at 1.79 A.
Proteins. 82:1708-20.
9. Liu, S ?.,
Zhang, C ?., Li, N., Niu, B., Liu, M., Liu, X., Wei, T., Zhu, D., Huang, Y., Xu, S., and Gu, L. (2012). Structural insight into the ISC domain of VibB from Vibrio cholerae at atomic resolution: a snapshot just before the enzymatic reaction.
Acta Crystallogr D Biol Crystallogr. 68:1329-38.
10. Li, N.,
Zhang, C., Li, B., Liu, X., Huang, Y., Xu, S., and Gu, L. (2012). Unique iron coordination in iron-chelating molecule vibriobactin helps Vibrio cholerae evade mammalian siderocalin-mediated immune response.
J Biol Chem. 287:8912-8919.

