研究方向
癌癥的治療是我國乃至全球經(jīng)濟(jì)社會發(fā)展所面臨的嚴(yán)峻挑戰(zhàn)。相比于傳統(tǒng)療法,近年來興起的免疫治療采用了新的策略,并取得了突破性的進(jìn)展。然而,目前僅有小部分腫瘤患者能夠從免疫治療中獲益,且腫瘤針對治療產(chǎn)生的耐藥時有發(fā)生。如何改進(jìn)現(xiàn)有免疫療法的效果,成為亟待解決的問題。本課題組綜合運(yùn)用小鼠動物模型、抗體工程技術(shù)、高通量篩選等方法與手段,聚焦研究腫瘤微環(huán)境中的免疫調(diào)節(jié)機(jī)制,以及它們在免疫治療中的功能與應(yīng)用。具體研究方向包括:1)篩選與鑒定腫瘤發(fā)生與轉(zhuǎn)移過程中的驅(qū)動因子,并研發(fā)新的免疫治療策略;2)研究腫瘤微環(huán)境中的免疫抑制與免疫激活調(diào)控機(jī)制,以及它們在免疫治療中的作用;3)設(shè)計與研發(fā)新的可用于腫瘤免疫治療的下一代抗體藥物。
科學(xué)貢獻(xiàn)
我們對腫瘤免疫治療過程中,腫瘤內(nèi)浸潤的T細(xì)胞(TILs)功能做了系統(tǒng)的研究。我們的研究證明,通過促進(jìn)T細(xì)胞浸潤、增強(qiáng)樹突狀細(xì)胞所介導(dǎo)的抗原交叉遞呈,能夠有效地提高治療的有效性,并有希望打破腫瘤的耐藥性。基于這些研究,我們研發(fā)了若干種可以有效提高腫瘤內(nèi)淋巴細(xì)胞浸潤或T細(xì)胞活化的下一代抗體藥物,并且證明這些藥物能夠打破腫瘤對于免疫檢查點(diǎn)阻斷療法的耐藥性。我們的研究為臨床上提高腫瘤免疫治療的有效性、以及應(yīng)對腫瘤耐藥性提供了新的潛在方案。
主要研究成果
1. 我們對腫瘤免疫治療過程中,腫瘤浸潤T細(xì)胞(TILs)的功能進(jìn)行了系統(tǒng)的研究。我們的工作證明,通過促進(jìn)T細(xì)胞浸潤、增強(qiáng)樹突狀細(xì)胞所介導(dǎo)的抗原遞呈,能夠有效地提高治療的效率,并有希望克服腫瘤的耐藥性。基于這些研究,我們研發(fā)了多種可以提高腫瘤內(nèi)淋巴細(xì)胞浸潤或T細(xì)胞活化的下一代抗體藥物,并且證明這些藥物能夠克服腫瘤對于免疫檢查點(diǎn)阻斷療法的耐藥性(Cancer Cell, 2016; Nat Commun, 2018)。這些研究為臨床上提高腫瘤免疫治療的有效率、以及應(yīng)對腫瘤耐藥性提供了新的潛在方案。
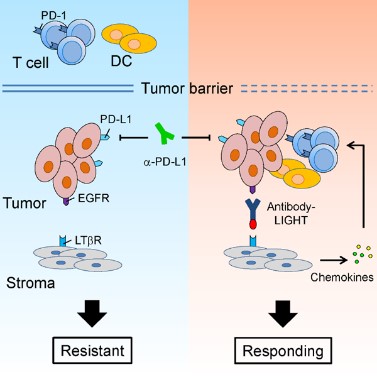
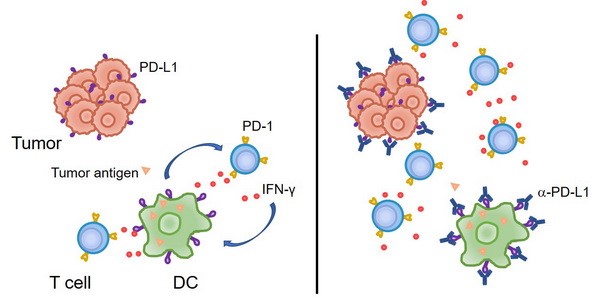
2. 傳統(tǒng)的觀點(diǎn)認(rèn)為,腫瘤細(xì)胞上表達(dá)的PD-L1與T細(xì)胞的PD-1相互作用,從而抑制T細(xì)胞反應(yīng)、并導(dǎo)致了腫瘤的免疫逃逸。然而我們的研究發(fā)現(xiàn),至少在某些情況下,髓系細(xì)胞、特別是樹突狀細(xì)胞上所表達(dá)的PD-L1在免疫治療中起到了更加關(guān)鍵的作用。腫瘤微環(huán)境通過特定機(jī)制誘導(dǎo)樹突狀細(xì)胞上調(diào)PD-L1,進(jìn)而抑制了T細(xì)胞與抗腫瘤免疫反應(yīng)(Nat Commun, 2020; J Clin Invest, 2018)。在臨床上,腫瘤患者的髓系細(xì)胞PD-L1表達(dá)水平可以作為生物標(biāo)志物,更好地預(yù)測免疫檢查點(diǎn)阻斷治療的預(yù)后。
榮譽(yù)和獎項(xiàng)
2019年Bayer Investigator Award
2014年Irvington Postdoctoral Fellowship, Cancer Research Institute (New York)
2012年北京生命科學(xué)研究院和賽諾菲巴斯德生物醫(yī)學(xué)杰出研究生獎
代表性論文
(#co-first author, *corresponding author)
1. Xiao K#, Zhang S#, Peng Q#, Du Y#, Yao X, Ng I-I, Tang H*. PD-L1 protects tumor-associated dendritic cells from ferroptosis during immunogenic chemotherapy, Cell Rep, 2024, 43: 114868.
2. Ng I-I#, Zhang J#, Tian T#, Peng Q#, Huang Z, Xiao K, Yao X, Ng L*, Zeng J*, Tang H*. Network-based screening identifies sitagliptin as an antitumor drug targeting dendritic cells, J Immunother Cancer, 2024, 12 (3): e008254.
3. Liu C#, Xiao K#, Yu C#, Lei Y#, Lyu K, Tian T, Zhao D*, Zhou F*, Tang H*, Zeng J*. A probabilistic knowledge graph for target identification. PLoS Comput Biol, 2024, 20 (4): e1011945.
4. Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu Y-X, Tang H#. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020, 11: 4835.
5. Zuo H, Yang D, Yang Q, Tang H, Fu Y-X, Wan Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat Commun. 2020, 11: 1578.
6. Tang H#, Liang Y#, Anders R, Taube J, Qiu X, Mulgaonkar A, Liu X, Harrington S, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Peng H, Dong H, Fu Y-X. PD-L1 on host cells is essential for tumor regression mediated by PD-L1 blockade. J Clin Invest. 2018, 128: 580-588.
7. Tang H*, Qiu X, Timmerman C, Fu Y-X. Targeting tertiary lymphoid structures for tumor immunotherapy. Methods Mol Biol. 2018, 1845: 275-286.
8. Tang H, Fu Y-X. Immune Evasion in Tumor’s Own Sweet Way. Cell Metab. 2018, 27: 945-946.
9. Liang Y#, Tang H#*, Guo J#, Qiu X, Ren Z, Bian Y, Dong H, Peng H*, Fu Y-X*. Targeting type I interferon into tumor by anti-PD-L1 creates feedforward antitumor responses to overcome innate and adaptive resistance. Nat Commun. 2018, 9: 4586.
10. Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu Y-X, Spiotto M, Weichselbaum R, Lin W. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018, 2: 600-610.
11. Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, He L, Chen Y, Chen H, Luo W, Lu Z, Xie J, Churchill H, Xu Y, Zhou Z, Wu G, Yu C, John S, Hirayasu K, Nguyen N, Liu X, Huang F, Li L, Deng H, Tang H, Sadek AH, Zhang L, Huang T, Zou Y, Chen B, Zhu H, Arase H, Xia N, Jiang Y, Collins R, You MJ, Homsi J, Unni N, Lewis C, Chen GQ, Fu YX, Liao XC, An Z, Zheng J, Zhang N, Zhang CC. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018, 562: 605-609.
12. Zhang Y, Kim T-J, Wroblewska J, Tesic V, Upadhyay V, Weichselbaum R, Tumornoy A, Tang H, Guo X, Tang H, Fu Y-X. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell Mol Immunol. 2017, 14: 1-13.
13. Tang H*, Zhu M, Qiao J, Fu Y-X*. Lymphotoxin signaling in tertiary lymphoid structures and immunotherapy. Cell Mol Immunol. 2017, 14: 809-818.
14. Qiao J, Tang H, Fu Y-X. DNA sensing and immune responses in cancer therapy. Curr Opin Immunol. 2017, 45: 16-20.
15. Wroblewska J, Zhang Y, Tang H, Guo X, Nagler C, Fu Y-X. Cutting Edge: Lymphotoxin Signaling Is Essential for Clearance of Salmonella from the Gut Lumen and Generation of Anti-Salmonella Protective Immunity. J Immunol. 2017, 198: 55-60.
16. Tang H, Wang Y, Chlewicki L, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu Y-X. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016, 29 (3): 285-296.
17. Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016, 370: 85-90.
18. Okwor I, Xu G, Tang H, Liang Y, Fu Y-X, Uzonna J. Deficiency of CD40 reveals an important role for LIGHT in anti-Leishmania immunity. J Immunol. 2015, 195: 194-202.
19. Li C, Yang Z, Du Y, Tang H, Chen J, Hu D, Fan Z. BCMab1, A monoclonal antibody against aberrantly glycosylated integrin a3b1, has potent antitumor activity of bladder cancer in vivo. Clin Cancer Res, 2014, 20: 4001-4013.
20. Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol.2012, 188: 824-831.
21. Yang Z, Tang H, Huang H, Deng H. RTA promoter demethylation and histone acetylation regulation of murine gamma-herpesvirus 68 reactivation. PLoS One. 2009, 4 (2): e4556.

