研究方向
癌癥的治療是我國乃至全球經濟社會發(fā)展所面臨的嚴峻挑戰(zhàn)。相比于傳統(tǒng)療法,近年來興起的免疫治療采用了新的策略,并取得了突破性的進展。然而,目前僅有小部分腫瘤患者能夠從免疫治療中獲益,且腫瘤針對治療產生的耐藥時有發(fā)生。如何改進現(xiàn)有免疫療法的效果,成為亟待解決的問題。本課題組綜合運用小鼠動物模型、抗體工程技術、高通量篩選等方法與手段,聚焦研究腫瘤微環(huán)境中的免疫調節(jié)機制,以及它們在免疫治療中的功能與應用。具體研究方向包括:1)篩選與鑒定腫瘤發(fā)生與轉移過程中的驅動因子,并研發(fā)新的免疫治療策略;2)研究腫瘤微環(huán)境中的免疫抑制與免疫激活調控機制,以及它們在免疫治療中的作用;3)設計與研發(fā)新的可用于腫瘤免疫治療的下一代抗體藥物。
科學貢獻
我們對腫瘤免疫治療過程中,腫瘤內浸潤的T細胞(TILs)功能做了系統(tǒng)的研究。我們的研究證明,通過促進T細胞浸潤、增強樹突狀細胞所介導的抗原交叉遞呈,能夠有效地提高治療的有效性,并有希望打破腫瘤的耐藥性。基于這些研究,我們研發(fā)了若干種可以有效提高腫瘤內淋巴細胞浸潤或T細胞活化的下一代抗體藥物,并且證明這些藥物能夠打破腫瘤對于免疫檢查點阻斷療法的耐藥性。我們的研究為臨床上提高腫瘤免疫治療的有效性、以及應對腫瘤耐藥性提供了新的潛在方案。
主要研究成果
1. 我們對腫瘤免疫治療過程中,腫瘤浸潤T細胞(TILs)的功能進行了系統(tǒng)的研究。我們的工作證明,通過促進T細胞浸潤、增強樹突狀細胞所介導的抗原遞呈,能夠有效地提高治療的效率,并有希望克服腫瘤的耐藥性。基于這些研究,我們研發(fā)了多種可以提高腫瘤內淋巴細胞浸潤或T細胞活化的下一代抗體藥物,并且證明這些藥物能夠克服腫瘤對于免疫檢查點阻斷療法的耐藥性(Cancer Cell, 2016; Nat Commun, 2018)。這些研究為臨床上提高腫瘤免疫治療的有效率、以及應對腫瘤耐藥性提供了新的潛在方案。
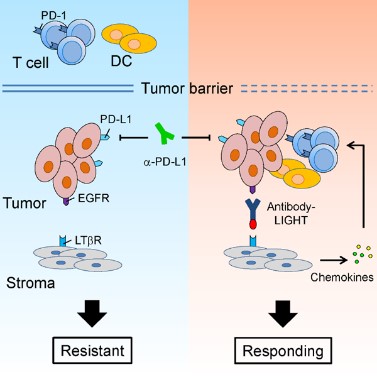
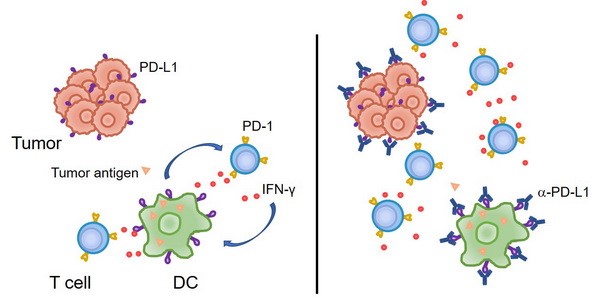
2. 傳統(tǒng)的觀點認為,腫瘤細胞上表達的PD-L1與T細胞的PD-1相互作用,從而抑制T細胞反應、并導致了腫瘤的免疫逃逸。然而我們的研究發(fā)現(xiàn),至少在某些情況下,髓系細胞、特別是樹突狀細胞上所表達的PD-L1在免疫治療中起到了更加關鍵的作用。腫瘤微環(huán)境通過特定機制誘導樹突狀細胞上調PD-L1,進而抑制了T細胞與抗腫瘤免疫反應(Nat Commun, 2020; J Clin Invest, 2018)。在臨床上,腫瘤患者的髓系細胞PD-L1表達水平可以作為生物標志物,更好地預測免疫檢查點阻斷治療的預后。
榮譽和獎項
2019年Bayer Investigator Award
2014年Irvington Postdoctoral Fellowship, Cancer Research Institute (New York)
2012年北京生命科學研究院和賽諾菲巴斯德生物醫(yī)學杰出研究生獎
代表性論文
(#co-first author, *corresponding author)
1. Xiao K#, Zhang S#, Peng Q#, Du Y#, Yao X, Ng I-I, Tang H*. PD-L1 protects tumor-associated dendritic cells from ferroptosis during immunogenic chemotherapy, Cell Rep, 2024, 43: 114868.
2. Ng I-I#, Zhang J#, Tian T#, Peng Q#, Huang Z, Xiao K, Yao X, Ng L*, Zeng J*, Tang H*. Network-based screening identifies sitagliptin as an antitumor drug targeting dendritic cells, J Immunother Cancer, 2024, 12 (3): e008254.
3. Liu C#, Xiao K#, Yu C#, Lei Y#, Lyu K, Tian T, Zhao D*, Zhou F*, Tang H*, Zeng J*. A probabilistic knowledge graph for target identification. PLoS Comput Biol, 2024, 20 (4): e1011945.
4. Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu Y-X, Tang H#. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020, 11: 4835.
5. Zuo H, Yang D, Yang Q, Tang H, Fu Y-X, Wan Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat Commun. 2020, 11: 1578.
6. Tang H#, Liang Y#, Anders R, Taube J, Qiu X, Mulgaonkar A, Liu X, Harrington S, Guo J, Xin Y, Xiong Y, Nham K, Silvers W, Hao G, Sun X, Chen M, Hannan R, Qiao J, Peng H, Dong H, Fu Y-X. PD-L1 on host cells is essential for tumor regression mediated by PD-L1 blockade. J Clin Invest. 2018, 128: 580-588.
7. Tang H*, Qiu X, Timmerman C, Fu Y-X. Targeting tertiary lymphoid structures for tumor immunotherapy. Methods Mol Biol. 2018, 1845: 275-286.
8. Tang H, Fu Y-X. Immune Evasion in Tumor’s Own Sweet Way. Cell Metab. 2018, 27: 945-946.
9. Liang Y#, Tang H#*, Guo J#, Qiu X, Ren Z, Bian Y, Dong H, Peng H*, Fu Y-X*. Targeting type I interferon into tumor by anti-PD-L1 creates feedforward antitumor responses to overcome innate and adaptive resistance. Nat Commun. 2018, 9: 4586.
10. Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu Y-X, Spiotto M, Weichselbaum R, Lin W. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018, 2: 600-610.
11. Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, He L, Chen Y, Chen H, Luo W, Lu Z, Xie J, Churchill H, Xu Y, Zhou Z, Wu G, Yu C, John S, Hirayasu K, Nguyen N, Liu X, Huang F, Li L, Deng H, Tang H, Sadek AH, Zhang L, Huang T, Zou Y, Chen B, Zhu H, Arase H, Xia N, Jiang Y, Collins R, You MJ, Homsi J, Unni N, Lewis C, Chen GQ, Fu YX, Liao XC, An Z, Zheng J, Zhang N, Zhang CC. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018, 562: 605-609.
12. Zhang Y, Kim T-J, Wroblewska J, Tesic V, Upadhyay V, Weichselbaum R, Tumornoy A, Tang H, Guo X, Tang H, Fu Y-X. Type 3 innate lymphoid cell-derived lymphotoxin prevents microbiota-dependent inflammation. Cell Mol Immunol. 2017, 14: 1-13.
13. Tang H*, Zhu M, Qiao J, Fu Y-X*. Lymphotoxin signaling in tertiary lymphoid structures and immunotherapy. Cell Mol Immunol. 2017, 14: 809-818.
14. Qiao J, Tang H, Fu Y-X. DNA sensing and immune responses in cancer therapy. Curr Opin Immunol. 2017, 45: 16-20.
15. Wroblewska J, Zhang Y, Tang H, Guo X, Nagler C, Fu Y-X. Cutting Edge: Lymphotoxin Signaling Is Essential for Clearance of Salmonella from the Gut Lumen and Generation of Anti-Salmonella Protective Immunity. J Immunol. 2017, 198: 55-60.
16. Tang H, Wang Y, Chlewicki L, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu Y-X. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016, 29 (3): 285-296.
17. Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016, 370: 85-90.
18. Okwor I, Xu G, Tang H, Liang Y, Fu Y-X, Uzonna J. Deficiency of CD40 reveals an important role for LIGHT in anti-Leishmania immunity. J Immunol. 2015, 195: 194-202.
19. Li C, Yang Z, Du Y, Tang H, Chen J, Hu D, Fan Z. BCMab1, A monoclonal antibody against aberrantly glycosylated integrin a3b1, has potent antitumor activity of bladder cancer in vivo. Clin Cancer Res, 2014, 20: 4001-4013.
20. Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol.2012, 188: 824-831.
21. Yang Z, Tang H, Huang H, Deng H. RTA promoter demethylation and histone acetylation regulation of murine gamma-herpesvirus 68 reactivation. PLoS One. 2009, 4 (2): e4556.

